Abstract
Introduction Immune thrombocytopenia (ITP) is an acquired autoimmune disease that is characterized by increased antibody-mediated platelet destruction, decreased megakaryopoiesis and impaired thrombopoiesis. An abnormal bone marrow microenvironment has been proposed to play an essential role in the progression of ITP [Stem Cells Dev, 2017]. Cytometry by Time of Flight (CyTOF) is a new analytic strategy that provides single-cell proteomics for enhanced cellular analysis, and describes the components of immune cell populations and the characteristics of new biomarkers. Here, we aim to provide a high-dimensional overview and summary of the changes in cell subsets and functions in the bone marrow of patients with ITP using CyTOF.
Methods A total of 40 patients diagnosed with ITP (21 men and 19 women; 23-71 years of age, median 47 years) were enrolled between July and October 2021. Based on the onset and progression of the disease, the ITP patients were classified into 3 subgroups. Group ITP-N1 included 15 newly diagnosed treatment-naïve patients ≤3 months after diagnosis, Group ITP-N2 included 10 patients with an ITP diagnosis of > 3 months who had not received any standard treatment (>3-12 months after diagnosis), and Group ITP-R included 15 patients who had received standard treatment but relapsed following remission. The healthy control (HC) group consisted of 10 donors who underwent hematopoietic stem cell transplantation. CyTOF panels were designed using 42 parameters that were measured at the single-cell level in the bone marrow of the ITP patients and HCs. The plasma levels of immune checkpoint regulators, such as PD-1, PD-L1, CTLA-4, TIM-3, LAG-3 and Galectin-9, were measured using a multianalyte flow assay kit (BioLegend).
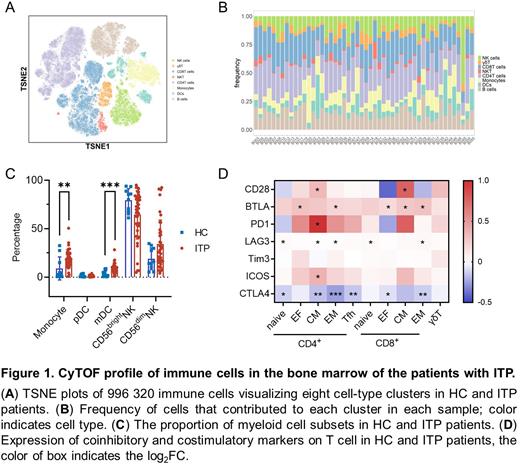
Results The platelet counts of enrolled ITP patients ranged from 1 to 96×109/L, with a median count of 25×109/L. Using CyTOF, the immune cells in the bone marrow were classified into 7 major subsets, including B cells, γδT cells, CD4+ T cells, CD8+ T cells, natural killer (NK) cells, dendritic cells (DCs), and monocytes.
The proportions of DCs and monocytes were significantly different between the ITP patients and HCs. The proportion of monocytes was observed to increase significantly in the bone marrow of the ITP patients. The proportion of the pDC subset of DCs showed a trend of being reduced in the ITP patients, while the proportion of mDCs was increased significantly in the ITP patients. NK cells can be divided into 2 subsets according to the expression of CD56, and these subsets exhibit different cytokine production patterns and cytotoxic functions. The proportion of CD56low NK cells was elevated and the proportion of CD56bright NK cells decreased significantly in ITP-R patients compared to HCs; similar results were not observed in the ITP-N1 or ITP-N2 groups. NK cells in bone marrow may play an important role in the relapse of ITP.
We further investigated the functional status of T cells in the ITP patients using a CyTOF panel. The results showed that the proportions of both CD4+ naïve and CD8+ naïve T cells were decreased in the bone marrow of the ITP patients. In particular, the proportion of CD8+ effector T cells was increased significantly in the ITP patients. Next, we analyzed coinhibitory and costimulatory receptor expression in each T-cell subpopulation. CTLA4 expression was observed to be reduced in CD8+ effector T cells and CD4+ Tfh cells. In addition to the changes in coinhibitory receptor expression, costimulatory molecules such as CD28 and ICOS were found to be expressed at higher levels in CD4+ central memory T cells. Combined with the soluble regulators in plasma, we found that the B7.2 levels in plasma was positively correlated with the cell surface expression of CD28 and negatively correlated with the expression of CTLA4 in T cells.
Conclusions This study provides a comprehensive overview of the human bone marrow immune characteristics of patients with ITP. Using CyTOF to conduct single-cell analyses, we mapped myeloid and T-cell subset phenotypes in particular and focused on the expression of coinhibitory molecules such as CTLA4. The different proportions of bone marrow immune cells and patterns of immune checkpoint molecule expression in different stages of ITP may provide novel insights into disease etiology and offer promising therapeutic targets for patients with ITP.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal